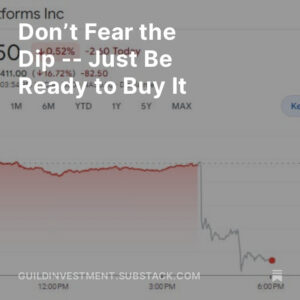
The stock market rally returned over the weekend as election results suggested the likelihood of a win for Presidential hopeful Joe Biden, the onset of a divided government, and the avoidance of prospective major tax increases. Stock markets seem to like gridlocked government. Fuel was added to the fire when Pfizer [NYSE: PFE] announced Phase 3 topline results from the trial of the Covid vaccine it is developing in collaboration with BioNTech [NASDQ: BNTX].
The topline data met the so-called “primary endpoint,” which (naturally) is to prevent Covid infection in those with no evidence of prior infection. Further, the company’s analysis suggested a 90% efficacy rate at seven days after the administration of the second of two doses in this regimen. There are many vaccines in development, some of them in advanced stages, but that efficacy rate sets a high bar for other contenders if it remains standing when the final trial analysis is completed. The next step will be for PFE/BNTX to seek an Emergency Use Authorization (EUA), which they will be able to do when they have two months of safety data to present. That will be in the third week of November.
Obviously, the main significance of this announcement is psychological. Markets were looking for a development that puts “return to normal” on the calendar in the foreseeable future, and the PFE/BNTX news gave some well-founded optimism that all the efforts of “Operation Warp Speed” would in fact bear fruit.
When it comes to the specifics of this vaccine, it’s important to note some caveats. Perhaps the most significant concerns storage. The vaccine requires storage at –70°C. Such conditions can be maintained only by specialized shipment and storage services, so the vaccine won’t be coming to your neighborhood pharmacy or doctor’s office any time soon. The EUA will enable the vaccination of frontline workers and particularly vulnerable populations. Although Herculean efforts will certainly be made to build the delivery structure out ahead of a national vaccination initiative, the difficulties suggest an opening for other vaccines with less onerous logistical requirements.
Currently, some other major vaccine candidates in development include those from Moderna [NASDAQ: MRNA], AstraZeneca [NYSE: AZN], Johnson & Johnson [NYSE: JNJ], Merck [NYSE: MRK], and Translate Bio [NASDAQ: TBIO]. MRNA’s and TBIO’s vaccines, like the PFE/NBRX vaccine, employ messenger RNA, a promising technology which we described in late 2018. Therefore there is some read-through from PFE’s success — although that read-through also includes some caution, since no mRNA therapeutic has yet been approved. Some other vaccine candidates have produced noteworthy adverse events; these considerations prompt a cooler analysis of prospects for a quick fix.
Besides logistics, and the novelty of the underlying technology of mRNA vaccines, there are other reasons to be somewhat cautious of the PFE news as an “all clear.” For example, when the study is complete, it will include evaluations of secondary endpoints — most notably, its effectiveness in reducing the severity of disease when people do develop symptoms, which is a key concern of physicians. Durability is another question, with many analysts expecting annual booster shots to be necessary. In short, there are plenty of reasons why this vaccine, as welcome as it is in many circles, will not be a simple silver bullet to end the pandemic in short order.
Additionally, there is the elephant in the room of public acceptance. Surveys suggest that Americans are rather skeptical of Covid vaccines; only about half of respondents indicate that they would seek to be vaccinated if one were available, and that number has fallen through the summer and fall as Covid mortalities have remained moderate in spite of rising case numbers. Still, other evidence suggests that public acceptance rises after the presentation of robust trial results.
Investment implications: Strong results from the Pfizer/BioNTech vaccine trial have ignited hopes for a “return to normal” some time next year, and led to a strong rally in the some of the stocks most damaged by the pandemic. While we think some caution is warranted on this vaccine specifically, the development does lend credence to the timeline of a widely available vaccine in 2021. It is likely that clearer results and more safety data will persuade a larger proportion of the U.S. population to accept the vaccine, in spite of currently expressed scepticism.